Coordination geometry: Difference between revisions
Officer781 (talk | contribs) →Table of coordination geometries: link to dodecahedral molecular geometry |
→top: Fixed typo Tags: canned edit summary Mobile edit Mobile app edit Android app edit |
||
| (40 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Term used in chemistry}} |
|||
The term '''coordination geometry''' is used in a number of related fields of chemistry and solid state chemistry/physics. |
|||
==Molecules== |
|||
{{main|Molecular geometry}} |
{{main|Molecular geometry}} |
||
The coordination geometry of an atom is the geometrical pattern |
The '''coordination geometry''' of an atom is the geometrical pattern defined by the atoms around the central atom. The term is commonly applied in the field of [[inorganic chemistry]], where diverse structures are observed. The coordination geometry depends on the number, not the type, of ligands bonded to the metal centre as well as their locations. The number of atoms bonded is the [[coordination number]]. |
||
| ⚫ | The geometrical pattern can be described as a [[polyhedron]] where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.<ref name = "IUCr">{{cite journal | title = Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types | journal = Acta Crystallogr. A | volume = 46 | pages = 1–11 | year = 1990 | doi = 10.1107/S0108767389008834 | author1 = J. Lima-de-Faria | author2 = E. Hellner | author3 = F. Liebau | author4 = E. Makovicky | author5 = E. Parthé | doi-access = free }}</ref> |
||
==Inorganic coordination complexes== |
|||
In the field of inorganic coordination complexes it is the geometrical pattern formed by the atoms in the [[ligand]]s that are [[coordination (bond)|bonded]] to the central atom in a [[molecule]] or a [[coordination complex]]. The geometrical arrangement will vary according to the number and type of ligands bonded to the metal centre, and to the coordination preference of the central atom, typically a metal in a [[coordination complex]]. The number of atoms bonded, (i.e. the number of [[sigma bonds|σ-bonds]] between central atom and ligands) is termed the [[coordination number]]. |
|||
| ⚫ | The geometrical pattern can be described as a [[polyhedron]] where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.<ref name = "IUCr">{{cite journal | title = Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types | journal = Acta Crystallogr. A | volume = 46 | pages = 1–11 | year = 1990 |doi = 10.1107/S0108767389008834 | author1 = J. Lima-de-Faria | author2 = E. Hellner | author3 = F. Liebau | author4 = E. Makovicky | author5 = E. Parthé | |
||
The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds ([[coordination number]]) can vary from two as high as 20 in Th(η |
The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds ([[coordination number]]) can vary from two in [[Potassium dicyanoargentate|{{chem2|K[Ag(CN)2]}}]] as high as 20 in [[Organothorium chemistry|{{chem2|Th(''η''^{5}\-C5H5)4}}]].<ref name = "Greenwood">{{Greenwood&Earnshaw}}</ref> |
||
One of the most common coordination geometries is [[octahedral molecular geometry|octahedral]], where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an [[octahedron]] if lines were drawn between the ligands. Other common coordination geometries are [[tetrahedral molecular geometry|tetrahedral]] and [[square planar molecular geometry|square planar]]. |
One of the most common coordination geometries is [[octahedral molecular geometry|octahedral]], where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an [[octahedron]] if lines were drawn between the ligands. Other common coordination geometries are [[tetrahedral molecular geometry|tetrahedral]] and [[square planar molecular geometry|square planar]]. |
||
| Line 19: | Line 14: | ||
==Table of coordination geometries== |
==Table of coordination geometries== |
||
A table of the coordination geometries encountered is shown below with examples of their occurrence in |
A table of the coordination geometries encountered is shown below with examples of their occurrence in complexes found as discrete units in compounds and [[coordination sphere]]s around atoms in crystals (where there is no discrete complex). |
||
{| class="wikitable" |
{| class="wikitable" |
||
!style="text-align: center;"|Coordination number !! Geometry !! !! Examples of discrete (finite) complex !! Examples in crystals |
!style="text-align: center;"|Coordination number !! Geometry !! !! Examples of discrete (finite) complex !! Examples in crystals (infinite solids) |
||
|- |
|- |
||
|style="text-align: center;"| 2 |
|style="text-align: center;"| 2 |
||
|style="text-align: center;"|[[linear molecular geometry|linear]] |
|style="text-align: center;"|[[linear molecular geometry|linear]] |
||
||[[Image:Linear-3D-balls.png|100px]] |
||[[Image:Linear-3D-balls.png|100px]] |
||
||Ag(CN) |
||{{chem2|[Ag(CN)2]−}} in {{chem2|K[Ag(CN)2]}} <ref name = "Wells">Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications {{ISBN|0-19-855370-6}}</ref> |
||
||Ag in [[silver cyanide]],<br />Au in AuI <ref name = "Greenwood"/> |
||Ag in [[silver cyanide]],<br />Au in AuI <ref name = "Greenwood"/> |
||
|- |
|- |
||
| Line 33: | Line 28: | ||
|style="text-align: center;"|[[trigonal planar molecular geometry|trigonal planar]] |
|style="text-align: center;"|[[trigonal planar molecular geometry|trigonal planar]] |
||
||[[Image:Trigonal-3D-balls.png|100px]] |
||[[Image:Trigonal-3D-balls.png|100px]] |
||
|| |
||{{chem2|[HgI3]−}}<ref name = "Greenwood"/> |
||
||O in [[titanium dioxide| |
||O in [[titanium dioxide|{{chem2|TiO2}}]] [[rutile]] structure<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|4 |
|style="text-align: center;"|4 |
||
|style="text-align: center;"|[[tetrahedral molecular geometry|tetrahedral]] |
|style="text-align: center;"|[[tetrahedral molecular geometry|tetrahedral]] |
||
||[[Image:Tetrahedral-3D-balls.png|100px]] |
||[[Image:Tetrahedral-3D-balls.png|100px]] |
||
|| |
||{{chem2|[CoCl4](2−)}}<ref name = "Greenwood"/> |
||
||Zn and S in [[zinc sulfide]], Si in |
||Zn and S in [[zinc sulfide]], Si in [[silicon dioxide]]<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|4 |
|style="text-align: center;"|4 |
||
|style="text-align: center;"|[[square planar molecular geometry|square planar]] |
|style="text-align: center;"|[[square planar molecular geometry|square planar]] |
||
||[[Image:Square-planar-3D-balls.png|100px]] |
||[[Image:Square-planar-3D-balls.png|100px]] |
||
|| |
||{{chem2|[AgF4]−}}<ref name = "Greenwood"/> |
||
||[[copper(II) oxide|CuO]]<ref name = "Wells"/> |
||[[copper(II) oxide|CuO]]<ref name = "Wells"/> |
||
|- |
|- |
||
| Line 51: | Line 46: | ||
|style="text-align: center;"|[[trigonal bipyramidal molecular geometry|trigonal bipyramidal]] |
|style="text-align: center;"|[[trigonal bipyramidal molecular geometry|trigonal bipyramidal]] |
||
||[[Image:Trigonal-bipyramidal-3D-balls.png|100px]] |
||[[Image:Trigonal-bipyramidal-3D-balls.png|100px]] |
||
|| |
||{{chem2|[SnCl5]−}}<ref name = "Wells"/> |
||
|| |
|| |
||
|- |
|- |
||
| Line 57: | Line 52: | ||
|style="text-align: center;"|[[square pyramidal molecular geometry|square pyramidal]] |
|style="text-align: center;"|[[square pyramidal molecular geometry|square pyramidal]] |
||
||[[Image:Square-pyramidal-3D-balls.png|100px]] |
||[[Image:Square-pyramidal-3D-balls.png|100px]] |
||
|| |
||{{chem2|[InCl5](2−)}} in {{chem2|[N(CH2CH3)4]2[InCl5]}}<ref name = "Greenwood"/> |
||
|| |
|| |
||
|- |
|- |
||
| Line 63: | Line 58: | ||
|style="text-align: center;"|[[octahedral molecular geometry|octahedral]] |
|style="text-align: center;"|[[octahedral molecular geometry|octahedral]] |
||
||[[Image:Octahedral-3D-balls.png|100px]] |
||[[Image:Octahedral-3D-balls.png|100px]] |
||
||Fe( |
||{{chem2|[Fe(H2O)6](2+)}}<ref name = "Greenwood"/> |
||
||Na and Cl in NaCl<ref name = "Wells"/> |
||[[Sodium|Na]] and [[chlorine|Cl]] in [[Sodium chloride|NaCl]]<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|6 |
|style="text-align: center;"|6 |
||
|style="text-align: center;"|[[trigonal prismatic molecular geometry|trigonal prismatic]] |
|style="text-align: center;"|[[trigonal prismatic molecular geometry|trigonal prismatic]] |
||
||[[Image:Prismatic TrigonalP.png|100px]] |
||[[Image:Prismatic TrigonalP.png|100px]] |
||
||[[Hexamethyltungsten|{{chem2|W(CH3)6}}]]<ref>{{Housecroft2nd|page=725}}</ref> |
|||
| ⚫ | |||
||As in [[nickel arsenide|NiAs]], Mo in [[molybdenum disulfide| |
||[[Arsenic|As]] in [[nickel arsenide|NiAs]], [[Molybdenum|Mo]] in [[molybdenum disulfide|{{chem2|MoS2}}]]<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|7 |
|style="text-align: center;"|7 |
||
|style="text-align: center;"|[[pentagonal bipyramidal molecular geometry|pentagonal bipyramidal]] |
|style="text-align: center;"|[[pentagonal bipyramidal molecular geometry|pentagonal bipyramidal]] |
||
||[[Image:Pentagonal-bipyramidal-3D-balls.png|100px]] |
||[[Image:Pentagonal-bipyramidal-3D-balls.png|100px]] |
||
|| |
||{{chem2|[ZrF7](3−)}} in {{chem2|[NH4]3[ZrF7]}}<ref name = "Wells"/> |
||
||Pa in [[Protactinium(V) chloride| |
||Pa in [[Protactinium(V) chloride|{{chem2|PaCl5}}]] |
||
|- |
|- |
||
|style="text-align: center;"|7 |
|style="text-align: center;"|7 |
||
|style="text-align: center;"|[[capped octahedral molecular geometry|capped octahedral]] |
|style="text-align: center;"|[[capped octahedral molecular geometry|capped octahedral]] |
||
||[[Image:Face-capped octahedron.png|100px]] |
||[[Image:Face-capped octahedron.png|100px]] |
||
||{{chem2|[MoF7]−}}<ref>{{cite journal | title = "Non-VSEPR" Structures and Bonding in d(0) Systems | first = Martin | last = Kaupp | journal = Angew Chem Int Ed Engl | year = 2001 | volume = 40 | issue = 1 | pages = 3534–3565 | doi = 10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-# | pmid = 11592184 }}</ref> |
|||
||[Ho<sup>III</sup>(PhCOCHCOPh)<sub>3</sub>(H<sub>2</sub>O)]<ref>Crystal and molecular structure of the heptacoordinate complex tris(diphenylpropanedionato)aquoholmium, Ho(PhCOCHCOPh)<sub>3</sub>.H<sub>2</sub>O, Zalkin A., Templeton D.H., Karraker D.G, Inorganic chemistry, 1969, 8, 12,2680 - 2684; {{doi|10.1021/ic50082a029}}</ref> |
|||
||La in [[lanthanum(III) oxide|A- |
||La in [[lanthanum(III) oxide|A-{{chem2|La2O3}}]] |
||
|- |
|- |
||
|style="text-align: center;"|7 |
|style="text-align: center;"|7 |
||
|style="text-align: center;"|[[capped trigonal prismatic molecular geometry|capped trigonal prismatic]] |
|style="text-align: center;"|[[capped trigonal prismatic molecular geometry|capped trigonal prismatic]] |
||
||[[Image:MonocappTrigPrism.CapRightps.png|100px]] |
||[[Image:MonocappTrigPrism.CapRightps.png|100px]] |
||
|| |
||{{chem2|[TaF7](2−)}} in {{chem2|K2[TaF7]}}<ref name = "Wells"/> |
||
|| |
|| |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|- |
|- |
||
|style="text-align: center;"|8 |
|style="text-align: center;"|8 |
||
|style="text-align: center;"|[[square antiprismatic molecular geometry|square antiprismatic]] |
|style="text-align: center;"|[[square antiprismatic molecular geometry|square antiprismatic]] |
||
||[[Image:Square-antiprismatic-3D-balls.png|100px]] |
||[[Image:Square-antiprismatic-3D-balls.png|100px]] |
||
|| |
||{{chem2|[TaF8](3−)}} in {{chem2|Na3[TaF8]}}<ref name = "Wells"/><br /> {{chem2|[Zr(H2O)8](4+)}} [[aqua complex]]<ref name="Persson2010">{{cite journal|last1=Persson|first1=Ingmar|title=Hydrated metal ions in aqueous solution: How regular are their structures?|journal=Pure and Applied Chemistry|volume=82|issue=10|year=2010|pages=1901–1917|issn=0033-4545|doi=10.1351/PAC-CON-09-10-22|doi-access=free}}</ref> |
||
||[[Thorium(IV) iodide]]<ref name = "Wells"/> |
||[[Thorium(IV) iodide]]<ref name = "Wells"/> |
||
|- |
|- |
||
| Line 105: | Line 94: | ||
|style="text-align: center;"|[[dodecahedral molecular geometry|dodecahedral]] <br />(note: whilst this is the term generally<br /> used, the correct term is "bisdisphenoid"<ref name = "Wells"/><br /> or "[[snub disphenoid]]" as this polyhedron is a [[deltahedron]]) |
|style="text-align: center;"|[[dodecahedral molecular geometry|dodecahedral]] <br />(note: whilst this is the term generally<br /> used, the correct term is "bisdisphenoid"<ref name = "Wells"/><br /> or "[[snub disphenoid]]" as this polyhedron is a [[deltahedron]]) |
||
||[[Image:snub disphenoid.png|100px]] |
||[[Image:snub disphenoid.png|100px]] |
||
||Mo(CN) |
||{{chem2|[Mo(CN)8](4−)}} in {{chem2|K4[Mo(CN)8]*2H2O}}<ref name = "Wells"/> |
||
||Zr in |
||Zr in {{chem2|K2[ZrF6]}}<ref name = "Wells"/> |
||
| ⚫ | |||
|style="text-align: center;"|8 |
|||
|style="text-align: center;"|[[bicapped trigonal prismatic molecular geometry|bicapped trigonal prismatic]] |
|||
||[[Image:Square face bicapped trigonal prism.png|100px]] |
|||
||{{chem2|[ZrF8](4−)}}<ref>{{cite journal | title = Eight-Coordination | author1 = Jeremy K. Burdett | author2 = Roald Hoffmann | author3 = Robert C. Fay | journal = [[Inorganic Chemistry (journal)|Inorganic Chemistry]] | year = 1978 | volume = 17 | issue = 9 | pages = 2553–2568 | doi = 10.1021/ic50187a041 }}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|- |
|- |
||
|style="text-align: center;"|8 |
|style="text-align: center;"|8 |
||
| Line 112: | Line 113: | ||
||[[File:Hexagonale bipiramide.png|100px]] |
||[[File:Hexagonale bipiramide.png|100px]] |
||
|| |
|| |
||
||N in [[lithium nitride| |
||N in [[lithium nitride|{{chem2|Li3N}}]]<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|8 |
|style="text-align: center;"|8 |
||
| Line 118: | Line 119: | ||
|| |
|| |
||
|| |
|| |
||
||Ni in [[nickel arsenide]], NiAs; 6 As neighbours |
||Ni in [[nickel arsenide]], NiAs; 6 As neighbours + 2 Ni capping<ref>David G. Pettifor, ''Bonding and Structure of Molecules and Solids'', 1995, Oxford University Press,{{ISBN|0-19-851786-6}}</ref> |
||
|- |
|- |
||
|style="text-align: center;"|8 |
|style="text-align: center;"|8 |
||
| Line 124: | Line 125: | ||
|| |
|| |
||
|| |
|| |
||
||Ca in |
||Ca in {{chem2|CaFe2O4}}<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"| |
|style="text-align: center;"|9 |
||
|style="text-align: center;"|trigonal prismatic |
|style="text-align: center;"|[[tricapped trigonal prismatic molecular geometry|tricapped trigonal prismatic]] |
||
| ⚫ | |||
|| |
|||
| ⚫ | |||
|| |
|||
|| |
||{{chem2|SrCl2*6H2O}}, Th in {{chem2|Rb[Th3F13]}}<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|9 |
|style="text-align: center;"|9 |
||
|style="text-align: center;"| |
|style="text-align: center;"|[[capped square antiprismatic molecular geometry|capped square antiprismatic]] |
||
| ⚫ | |||
| ⚫ | |||
||SrCl<sub>2</sub>.6H<sub>2</sub>O, Th in RbTh<sub>3</sub>F<sub>13</sub><ref name = "Wells"/> |
|||
| ⚫ | |||
| ⚫ | |||
|style="text-align: center;"|monocapped square antiprismatic |
|||
||[[Image:Monocapped square antiprism.png|100px]] |
||[[Image:Monocapped square antiprism.png|100px]] |
||
||[Th(tropolonate) |
||{{chem2|[Th(tropolonate)4(H2O)]}}<ref name = "Greenwood"/>{{cln|reason=What on this Earth is "tropolonate"??? Clear this jargon for an average mortal human being!|date=July 2023}} |
||
||La in |
||La in {{chem2|LaTe2}}<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|10 |
|style="text-align: center;"|10 |
||
|style="text-align: center;"|bicapped square antiprismatic |
|style="text-align: center;"|bicapped square antiprismatic |
||
|| |
|| |
||
||Th( |
||{{chem2|[Th(C2O4)4](2−)}}<ref name = "Greenwood"/> |
||
|| |
|| |
||
|- |
|- |
||
| Line 153: | Line 148: | ||
|| |
|| |
||
|| |
|| |
||
||Th in [Th |
||Th in {{chem2|[Th^{IV}(NO3)4(H2O)3]}} ({{chem2|NO3−}} is [[bidentate]])<ref name = "Greenwood"/> |
||
|| |
|| |
||
|- |
|- |
||
| Line 159: | Line 154: | ||
|style="text-align: center;"|[[icosahedron]] |
|style="text-align: center;"|[[icosahedron]] |
||
||[[Image:icosahedron.png|100px]] |
||[[Image:icosahedron.png|100px]] |
||
||Th in Th( |
||Th in {{chem2|[Th(NO3)6](2−)}} ion in {{chem2|Mg[Th(NO3)6]*8H2O}}<ref name = "Wells"/> |
||
|- |
|- |
||
|style="text-align: center;"|12 |
|style="text-align: center;"|12 |
||
|style="text-align: center;"|[[cuboctahedron]] |
|style="text-align: center;"|[[cuboctahedron]] |
||
||[[Image:Cuboctahedron.png|100px]] |
||[[Image:Cuboctahedron.png|100px]] |
||
||{{chem2|Zr^{IV}(''η''^{3}\−[BH4]4)}} |
|||
||Zr<sup>IV</sup>(η<sup>3</sup>−(BH<sub>4</sub>)<sub>4</sub>) |
|||
||atoms in [[periodic table (crystal structure)|fcc metals]] e.g. Ca<ref name = "Wells"/> |
||atoms in [[periodic table (crystal structure)|fcc metals]] e.g. Ca<ref name = "Wells"/> |
||
|- |
|- |
||
| Line 170: | Line 165: | ||
|style="text-align: center;"|anticuboctahedron ([[triangular orthobicupola]]) |
|style="text-align: center;"|anticuboctahedron ([[triangular orthobicupola]]) |
||
||[[Image:triangular orthobicupola.png|100px]] |
||[[Image:triangular orthobicupola.png|100px]] |
||
|| |
|| |
||
| ⚫ | |||
Ddryfdfyui |
|||
| ⚫ | |||
|- |
|- |
||
|style="text-align: center;"| |
|style="text-align: center;"|12 |
||
|style="text-align: center;"|bicapped [[hexagonal antiprism]]atic |
|style="text-align: center;"|bicapped [[hexagonal antiprism]]atic |
||
|| |
|| |
||
||U |
||{{chem2|U[BH4]4}}<ref name = "Greenwood"/> |
||
|} |
|} |
||
==Naming of inorganic compounds== |
==Naming of inorganic compounds== |
||
IUPAC have introduced the [[polyhedral symbol]] as part of their [[IUPAC nomenclature of inorganic chemistry 2005|IUPAC nomenclature of inorganic chemistry 2005 recommendations]] to describe the geometry around an atom in a compound.<ref name = "IUPAC inorganic">NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 ed. N. G. Connelly et al. RSC Publishing |
IUPAC have introduced the [[polyhedral symbol]] as part of their [[IUPAC nomenclature of inorganic chemistry 2005|IUPAC nomenclature of inorganic chemistry 2005 recommendations]] to describe the geometry around an atom in a compound.<ref name = "IUPAC inorganic">NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 ed. N. G. Connelly et al. RSC Publishing http://www.chem.qmul.ac.uk/iupac/bioinorg/</ref><br /> |
||
IUCr have proposed a symbol which is shown as a superscript in square brackets in the chemical formula. For example, |
IUCr have proposed a symbol which is shown as a superscript in square brackets in the chemical formula. For example, {{chem2|CaF2}} would be Ca<sup>[8cb]</sup>F<sub>2</sub><sup>[4t]</sup>, where [8cb] means cubic coordination and [4t] means tetrahedral. The equivalent symbols in IUPAC are ''CU''−8 and ''T''−4 respectively.<ref name = "IUCr"/> <br /> |
||
The IUPAC symbol is applicable to complexes and molecules whereas the IUCr proposal applies to crystalline solids. |
The IUPAC symbol is applicable to complexes and molecules whereas the IUCr proposal applies to crystalline solids. |
||
Latest revision as of 08:18, 26 January 2024
The coordination geometry of an atom is the geometrical pattern defined by the atoms around the central atom. The term is commonly applied in the field of inorganic chemistry, where diverse structures are observed. The coordination geometry depends on the number, not the type, of ligands bonded to the metal centre as well as their locations. The number of atoms bonded is the coordination number. The geometrical pattern can be described as a polyhedron where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.[1]
The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds (coordination number) can vary from two in K[Ag(CN)2] as high as 20 in Th(η5-C5H5)4.[2]
One of the most common coordination geometries is octahedral, where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an octahedron if lines were drawn between the ligands. Other common coordination geometries are tetrahedral and square planar.
Crystal field theory may be used to explain the relative stabilities of transition metal compounds of different coordination geometry, as well as the presence or absence of paramagnetism, whereas VSEPR may be used for complexes of main group element to predict geometry.
Crystallography usage[edit]
In a crystal structure the coordination geometry of an atom is the geometrical pattern of coordinating atoms where the definition of coordinating atoms depends on the bonding model used.[1] For example, in the rock salt ionic structure each sodium atom has six near neighbour chloride ions in an octahedral geometry and each chloride has similarly six near neighbour sodium ions in an octahedral geometry. In metals with the body centred cubic (bcc) structure each atom has eight nearest neighbours in a cubic geometry. In metals with the face centred cubic (fcc) structure each atom has twelve nearest neighbours in a cuboctahedral geometry.
Table of coordination geometries[edit]
A table of the coordination geometries encountered is shown below with examples of their occurrence in complexes found as discrete units in compounds and coordination spheres around atoms in crystals (where there is no discrete complex).
| Coordination number | Geometry | Examples of discrete (finite) complex | Examples in crystals (infinite solids) | |
|---|---|---|---|---|
| 2 | linear | [Ag(CN)2]− in K[Ag(CN)2] [3] | Ag in silver cyanide, Au in AuI [2] | |
| 3 | trigonal planar | 
|
[HgI3]−[2] | O in TiO2 rutile structure[3] |
| 4 | tetrahedral | 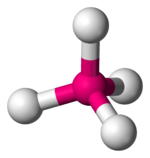
|
[CoCl4]2−[2] | Zn and S in zinc sulfide, Si in silicon dioxide[3] |
| 4 | square planar | 
|
[AgF4]−[2] | CuO[3] |
| 5 | trigonal bipyramidal | 
|
[SnCl5]−[3] | |
| 5 | square pyramidal | 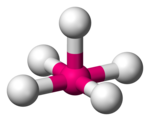
|
[InCl5]2− in [N(CH2CH3)4]2[InCl5][2] | |
| 6 | octahedral | 
|
[Fe(H2O)6]2+[2] | Na and Cl in NaCl[3] |
| 6 | trigonal prismatic | 
|
W(CH3)6[4] | As in NiAs, Mo in MoS2[3] |
| 7 | pentagonal bipyramidal | 
|
[ZrF7]3− in [NH4]3[ZrF7][3] | Pa in PaCl5 |
| 7 | capped octahedral | 
|
[MoF7]−[5] | La in A-La2O3 |
| 7 | capped trigonal prismatic | 
|
[TaF7]2− in K2[TaF7][3] | |
| 8 | square antiprismatic | 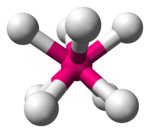
|
[TaF8]3− in Na3[TaF8][3] [Zr(H2O)8]4+ aqua complex[6] |
Thorium(IV) iodide[3] |
| 8 | dodecahedral (note: whilst this is the term generally used, the correct term is "bisdisphenoid"[3] or "snub disphenoid" as this polyhedron is a deltahedron) |

|
[Mo(CN)8]4− in K4[Mo(CN)8]·2H2O[3] | Zr in K2[ZrF6][3] |
| 8 | bicapped trigonal prismatic | 
|
[ZrF8]4−[7] | PuBr3[3] |
| 8 | cubic | Caesium chloride, calcium fluoride | ||
| 8 | hexagonal bipyramidal | 
|
N in Li3N[3] | |
| 8 | octahedral, trans-bicapped | Ni in nickel arsenide, NiAs; 6 As neighbours + 2 Ni capping[8] | ||
| 8 | trigonal prismatic, triangular face bicapped | Ca in CaFe2O4[3] | ||
| 9 | tricapped trigonal prismatic | 
|
[ReH9]2− in potassium nonahydridorhenate[2] [Th(H2O)9]4+ aqua complex[6] |
SrCl2·6H2O, Th in Rb[Th3F13][3] |
| 9 | capped square antiprismatic | 
|
[Th(tropolonate)4(H2O)][2][clarification needed] | La in LaTe2[3] |
| 10 | bicapped square antiprismatic | [Th(C2O4)4]2−[2] | ||
| 11 | Th in [ThIV(NO3)4(H2O)3] (NO−3 is bidentate)[2] | |||
| 12 | icosahedron | 
|
Th in [Th(NO3)6]2− ion in Mg[Th(NO3)6]·8H2O[3] | |
| 12 | cuboctahedron | 
|
ZrIV(η3-[BH4]4) | atoms in fcc metals e.g. Ca[3] |
| 12 | anticuboctahedron (triangular orthobicupola) | 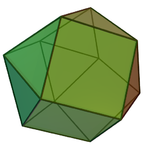
|
atoms in hcp metals e.g. Sc[3] | |
| 12 | bicapped hexagonal antiprismatic | U[BH4]4[2] |
Naming of inorganic compounds[edit]
IUPAC have introduced the polyhedral symbol as part of their IUPAC nomenclature of inorganic chemistry 2005 recommendations to describe the geometry around an atom in a compound.[9]
IUCr have proposed a symbol which is shown as a superscript in square brackets in the chemical formula. For example, CaF2 would be Ca[8cb]F2[4t], where [8cb] means cubic coordination and [4t] means tetrahedral. The equivalent symbols in IUPAC are CU−8 and T−4 respectively.[1]
The IUPAC symbol is applicable to complexes and molecules whereas the IUCr proposal applies to crystalline solids.
See also[edit]
References[edit]
- ^ a b c J. Lima-de-Faria; E. Hellner; F. Liebau; E. Makovicky; E. Parthé (1990). "Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types". Acta Crystallogr. A. 46: 1–11. doi:10.1107/S0108767389008834.
- ^ a b c d e f g h i j k l Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c d e f g h i j k l m n o p q r s t u v Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 725. ISBN 978-0-13-039913-7.
- ^ Kaupp, Martin (2001). ""Non-VSEPR" Structures and Bonding in d(0) Systems". Angew Chem Int Ed Engl. 40 (1): 3534–3565. doi:10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-#. PMID 11592184.
- ^ a b Persson, Ingmar (2010). "Hydrated metal ions in aqueous solution: How regular are their structures?". Pure and Applied Chemistry. 82 (10): 1901–1917. doi:10.1351/PAC-CON-09-10-22. ISSN 0033-4545.
- ^ Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi:10.1021/ic50187a041.
- ^ David G. Pettifor, Bonding and Structure of Molecules and Solids, 1995, Oxford University Press,ISBN 0-19-851786-6
- ^ NOMENCLATURE OF INORGANIC CHEMISTRY IUPAC Recommendations 2005 ed. N. G. Connelly et al. RSC Publishing http://www.chem.qmul.ac.uk/iupac/bioinorg/